μCheck - a smart system for online monitoring of bacterial cell size in bioprocesses
The μCheck system is used for online monitoring of cell size during the growth of bacteria in suspension culture. Measurement of cell concentration and biomass is also integrated into the μCheck system. The μCheck is suitable for real-time analysis of bacterial cells and chains in the size range 0.8 μm to 40 μm. Analysis is performed in flow-through mode without sampling, directly in the growth medium under sterile conditions.

The multiparametric system is designed for short- or long-term cultivation of bacterial species such as E. coli, Lactobacillus sp., Bifidobacterium sp., Corynebacterium sp., Ralstonia, Bacillus sp., Listeria sp., and many others. Continuous analysis with μCheck can be used for all types of cultivation methods (anaerobic, aerobic, batch, fed-batch)
A μCheck-D version with integrated dilution function is available as a special model for aerobic high-cell density cultivations. Due to the integrated dilution procedure and the optimised measuring technique, the analysis system is able to determine optical densities above 300 in real-time.
The μCheck system can be connected to any culture vessel or bioreactor using pre-fabricated sterile disposable tubing sets. μCheck has an integrated syringe pump and valves for precise sequencing of measurements and intermediate rinsing of the measurement zone. The aseptic design of the fluidic components and the closed sample loop without the necessity to draw a sample and interfer with the process.
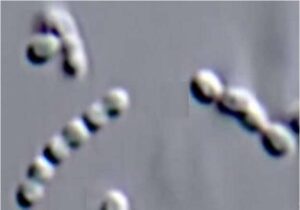
The μCheck system is almost maintenance-free. Due to its small size, it can be placed flexibly in the process environment. For connection to the bioreactor, the user has the proven connectors (sterilisable or disposable) and the bypass module at his disposal. Thanks to the structured design and the simple operating concept, routine analysis can be carried out after only a short instruction
μCheck can be operated as a stand-alone system or via a compact PC using the supplied software. For use with a PC, the unit is connected to the PC by means of communication interfaces (USB, Bluetooth). In addition, the measurement data is automatically stored on an internal memory card and can be retrieved separately if required. The μCheck software is suitable for parallel operation of up to four μCheck units. A tested compact PC with pre-installed software for visualisation and data management will be supplied.

For validation and routine verification of the measurement system, reference samples of bacterial cultures are available. The pre-tested batches of starter and probiotic cultures are long-time stable when stored. The average cell size (length) of these reference cultures is in the range between 2.0 and 6.0 μm. The μCheck optical measurement unit can also be easily validated using the OD standards supplied.
Areas of application

- Production of recombinant proteins and other biosubstances
- Production of starter cultures, probiotic and competent cells
- Development and production of vaccines, infection biology
- High-cell density fermentation processes
- Quality assurance and process validation of bacterial cultivations
- Long-term studies of bacterial evolution
- Many other fermentation strategies

Advantages
- Automatic, real-time determination of
– Cells and cells chain size
– Cells concentration
– Biomass (via OD measurement) - Online analysis directly in the growth environment
- Acquisit of a large numbers of cells for statistically validated
information - Smart system, easy-to-use by routine cultivations
- Suitable for culture development, monitoring and prediction
- Selection of optimal growth media and feeding strategy
- Suitable for batch, fed-batch, anaerobic and aerobic cultivation
processes - Operating with small, bench and pilot scale bioreactors
- Ideal for the use in parallel processes
- Excellent price/performance ratio

Functional specifications
μCheck classic
- Measurement of cell suspensions in transparent liquid media
- Measurement directly in growth media without dilution or
reagent addition - Sterile & aseptic measurement, easy handling
- Designed for rod-shaped bacteria, single cells and cell chains
- Detection range of cell (cell chains) length: 0.8 – 40 μm
(< 0.8 and > 40 μm variants on request) - Range of operation for the OD of a cell suspension: 0.2 – 40.0
(lower OD ranges available on request, for OD ranges > 40
the μCheck-D system is recommended) - Type of measurable microorganisms: bacteria like E. coli, Lactobacillus,
Bifidobacterium, Lactococcus, Streptococcus, Corynebacteruim,
Ralstonia, Bacillus sp., Listeria, and many others - Minimum suspension volume: 4 mL (lower volumes on
request). Maximum volume: > m³ - Minimum recommended cell concentration: 0.2 x 109 cells/mL
(version for lower concentrations on request) - Validation and routine testing of measurement system
A special design of the sample lines, connections and optical device ensures a sterile connection to the
cultivation volume and contamination-free operation. At the end of the bioprocess, the bypass line is
easy to decontaminate and dispose properly.

μCheck-D – a system with an automatic dilution function, designed for high-cell-density fermentations
- OD Operating range for cell suspension: 0.2 – 300
- Sample dilution factor > 4.5 (adjustable)
- Automatic dilution directly in the syringe plunger, no special
mixing camera required - High dilution reproducibility
- Analysis software automatically calculates OD and cell concentration
according to the dilution factor - Automated sampling, aseptic mode of operation
- Recommended starting OD for operation in dilution mode:
approx. 1.0 - Minimum sample volume used per measurement: 0.3 mL
- Consumption of diluent solution: approx. 300 mL in 24 h operation
(at 10 min sampling interval) - Measurement frequency freely selectable

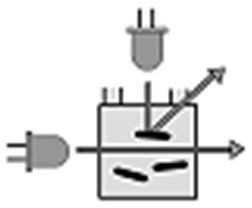
Bypass module is used to remove bubbles from the fermentation
solution. Can also be used for feeding fresh samples if the distance
between bioreactor and uCheck is long.
- Stand-alone unit for operation with a μCheck system
- Sterile connectors and sample lines, easy handling
- Includes compact peristaltic pump with adjustable flow rate
(3 – 20 mL/min) - Separation unit: glass vial with manifold head, retention
volume 1.5 or 3 mL
Technical specifications μCheck
Optical measurement
Areas of application

- Wavelength: 620 nm
- Maintenance-free LED technology
- Sterilisable and removable optical flow cell with a protective
holder - Optical thickness of optic al flow cell: < 1.5 mm
- Optimized for use in bacterial suspensions
- Designed to measure the orthogonal component of bacterial
cell orientation - Recording interval: 1 measurement per 0.25 s
- Accuracy: ± 0.1% (approx. ± 0.01 at low OD)
Integrated syringe pump & valves
- Integrated mini syringe pump and valves, simple handling
- Continuous, high-precision sample feeding
- Disposable syringe (working volume 1 mL), pharmaceutical
version, easy to install - Long disposable syringe lifetime (over 16000 cycles, approx.
20 operating days) - Media-separated miniature pinch valves, easy and aseptic bypass
tubing mounting
Flexible bypass loop
- Prefabricated sterile or sterilisable flow tubes (silicon, ID 1.3
and 1.5 mm) and Luer-Lock connectors - Disposable T-manifold, pharmaceutical version
- Residence volume in bypass loop: approx. 0.8 mL
- Safe, sterile connection to optical flow cell
- Designed for easy and aseptic connection to the culture vessel
Control panel and display
- Selector switch for ‘Run’, ‘Stop’ and ‘Pump’ operating modes
- Selection of the “Calibration” or “Measurement” mode with variable
/ adaptive or fixed duration of the relaxation phase - Selection of fixed cycle time (duration) in steps from 5 sec to
320 sec - Setting of “pumping down” of the dead volume in the feeding
line - Setting of the waiting time between the measuring cycles:
0 – 10 min - Integrated 2.2″ TFT LCD display panel, display: system status,
measurement signal history, calibration and current measured
values (OD, size, cell concentrations)
Software μCheck for easy set-up, real-time data visualisation and process data analysis
- MS Windows-based software package for signal recording,
visualisation and data management - Fast and accurate data recording
- Real-time data monitor
- Direct comparison between actual and archived data
- Several reporting functions
- Data Export to MS Excel and ASCII (Origin, PlotIT, etc.)
- Software designed for parallel operation of up to four μCheck
units
Validation
For validation and routine verifying of the measuring system, reference cultures (storage stable bacterial
strains) and optical reference standards (OD standards) are available.
Reference cultures
- Sterile packaged, storage stable, bacteria strain concentrates,
used in the dairy industry - Bacteria cultures: cells, chain size (length) from 2.0 to 6.0 μm
(average) - Test suspension is easy to prepare: mix culture concentrates
with diluent - Starter or protective cultures, industrial producers Chr. Hansen,
DuPont/Danisco, etc. - Tested batches of Brevibacterium linens, Lactococcus lactis,
Lactobacillus casei, Streptococcus thermophilus, Lactobacillus
rhamnosus, Lactobacillus helveticus. Others strains on request - Package size: 2 – 8 g, can be used in portions for multiple test
runs - Concentrates, minimum concentration range: 2 – 4*1011
CFU/g*
*- according to the manufacturer’s specifications
Optical reference standards
- Two optical grey filters in round holders
- Absorbance values (OD) at 620 nm: 0.74 and 1.48
- Easy verification of optical unit and optical linearity
Device configurations for various cultivation methods
| Features | uCheck classic | uCheck classic & bypass module* | uCheck-D & bypass module* |
|---|---|---|---|
| Cultivation methods | anaerobic & low gassing | aerobic | high cell density cultivation |
| OD range (suspension) | 0.2 – 40 | 0.2 – 40 | 0.2 – > 300 |
| Continuous, real-time measurement | ✓ | ✓ | ✓ |
| Sampling free | ✓ | ✓ | No (sample usage 0.3 mL per measurement) |
| Online & Offline mode | ✓ | ✓ | automatic offline operation |
| Aseptic processing design | ✓ | ✓ | ✓ |
| Minimum suspension volume required for measurement | 4 mL | 8 mL | 40 mL** |
| Dimensions (required desktop area, WxD) | 120 x 230 mm |
240 x 230 mm |
340 x 230 mm*** |
* – bubble separator function
** – for biomass concentration of OD 1.0 and higher
*** – flexible positioning of dilution and waste bottles
| Electrical characteristics
External power supply:
Communication / Data transfer
|
Dimensions (L x W x H) and weight
*- height: 20.5 cm with installed single-use syringe
Environment
|
Scope of delivery, options and consumables
Included in Delivery (basic version)
|
Optional
Consumables
Terms of delivery
|
Application examples
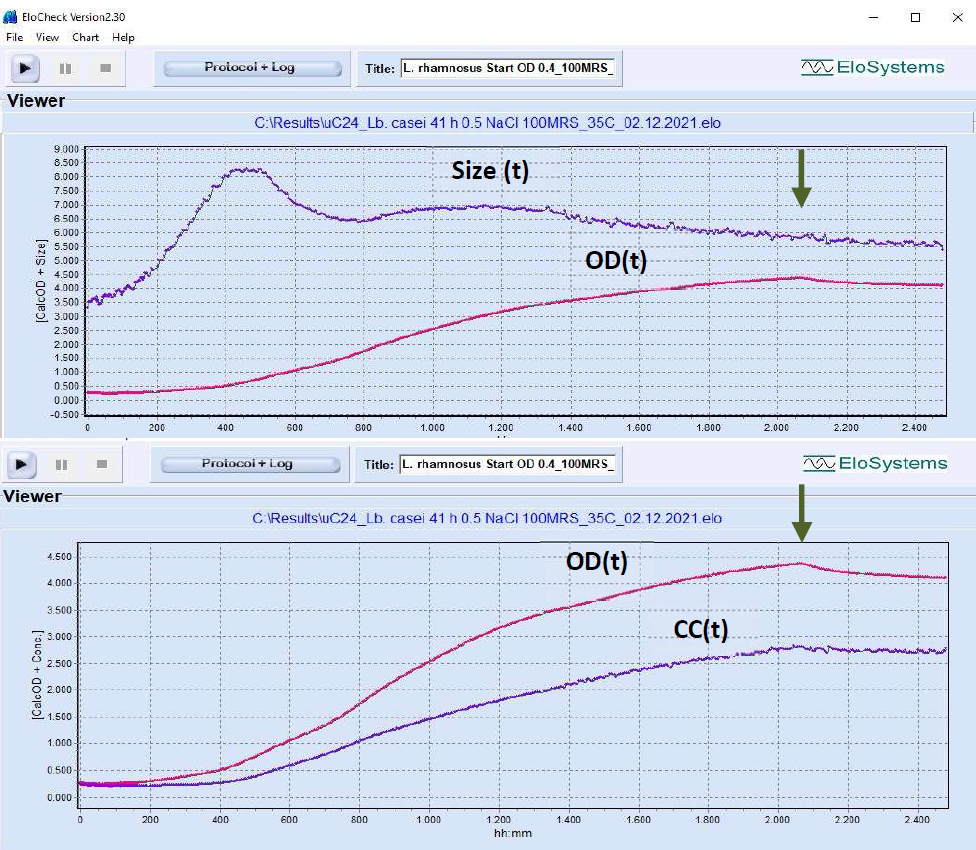
Fig. 1: Growth profile of Lactobacillus
casei (probiotic strain Lc-11®,
DuPont/Danisco) without pH control.
Cutivation volume: 5 mL. Growth conditions: 100% MRS-medium + 0.5 g/L
NaCl, anaerobic, T=35°C, final
pH=4.1. Variation of cell, cell chain
length: 3.5 µm inoculum, 8.3 µm max,
5.5 µm at the end of the cultivation. OD
– optical density at 620 nm. Cell concentration: 0.28*E+09 cells/ml at inoculation; final concentration:
2.58*E+09 cells/ml. Total cultivation
duration: 40.6 h. Arrow – time of glucose consumption.
[Device uCheck-24, number of measuring
cycles: 820]
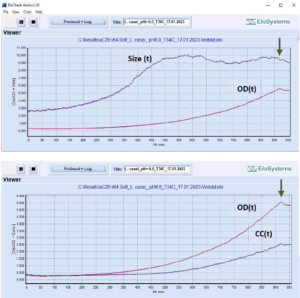
Fig. 2: Growth profile of Lactobacillus
casei (probiotic strain Lc-11®,
DuPont/Danisco) at controlled
pH=6.0. Cutivation volume: 5 mL.
Growth conditions: 100% MRS-medium,
anaerobic, T=35°C. Variation of
cell, cell chain length: 2.8 μm inoculum,
9.9 μm max, 9.0 μm at the end
of the cultivation. OD – optical density
at 620 nm. Cell concentration:
0.38*E+09 cells/mL at inoculation; final
concentration: 2.50*E+09
cells/mL. Total cultivation duration: 16
h. Arrow – time of glucose consumption.
[Device uCheck-26, number of measuring
cycles: 320]
Size (t), CC(t), OD(t): changes in cell, cell chain length (average), cell concentration and optical density
(as biomass) during growth.
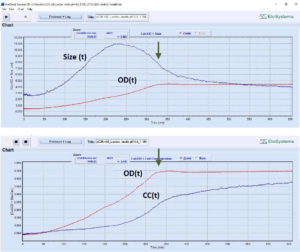
Fig. 3: Growth profile of Lactococcus
lactis (probiotic strain Ll-23,
DuPont/Danisco) at controlled
pH=6.0. Cutivation volume: 15 mL.
Growth conditions: 100% MRS-medium,
anaerobic, T=30°C. Variation of
cell, cell chain length: 2.7 μm inoculum,
10.9 μm max, 3.9 μm at the
end of the cultivation. OD – optical density
at 620 nm. Cell concentration
(CC): 0.49*E+09 cells/mL at inoculation;
final concentration: 3.75*E+09
cells/mL. Total cultivation duration: 11
h. Arrow – time of glucose consumption.
[Device uCheck-26, number of measuring
cycles: 220]
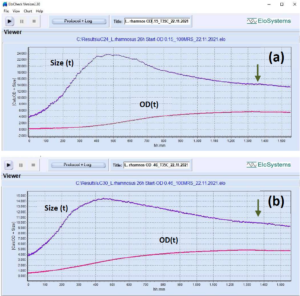
Fig. 4: Parallel analysis of the growth of
Lactobacillus rhamnosus (strain GG,
ATCC 53103, DuPont/Danisco) at
various inoculum sizes.
Sample (a) initial OD=0.15.
Sample (b) initial OD=0.46.
Monitoring of two identical vials, cutivation
volume: 5 mL. Growth conditions:
100% MRS medium, anaerobic, T=
35°C, without pH control, final pH=4.05
(both samples).
Variation of cell, cell chain length: (a)
3.9 μm inoculum, 23.9 μm max, 13.8
μm final;
(b) 3.9 μm inoculum, 14.4 μm max, 9.2
μm final.
Final OD and cell concentration:
(a) OD= 5.8; CC= 1.9*E+09 cells/mL,
(b) OD= 4.9; CC= 2.2*E+09 cells/mL.
Total cultivation duration: approx. 26 h.
Arrow – time of glucose consumption.
[Devices uCheck-24 and uCheck-30 running
in parallel, number of measuring
cycles: 793, both samples]
Further examples concerning the influence of temperature, pH, medium composition, additives,
growth or inhibitory factors on the growth of bacterial cultures are available on request. Studies on
adaptation mechanisms of Lactobacillus, Bifidobacterium and others in response to environmental
stress (heat, cold, acid, osmotic stress, freezing/thawing, starvation) are also possible.